

Stoichiometry - Engineering
Q1: Which equation is not an equation of state ?A PV = RT + B/V + y/V2 + ....
B (P + a/V2)(V-b) = RT
C 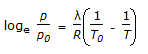
D 
ANS:C - The equation that is not an equation of state is: 𝑃𝑉=𝑅𝑇+𝐵𝑉+𝑦𝑉2+…PV=RT+VB+V2y+… This equation is known as the van der Waals equation, which is a modification of the ideal gas law to account for the finite size of gas molecules and the attractive forces between them. While it describes the behavior of real gases more accurately than the ideal gas law, it is not considered an equation of state in its standard form. The second equation: (𝑃+𝑎𝑉2)(𝑉−𝑏)=𝑅𝑇(P+V2a)(V−b)=RT is the van der Waals equation of state, which is an equation that describes the state of a system under given conditions. |


For help Students Orientation
Mcqs Questions
One stop destination for examination, preparation, recruitment, and more. Specially designed online test to solve all your preparation worries. Go wherever you want to and practice whenever you want, using the online test platform.

