

Stoichiometry - Engineering
Q1: Which of the following is the Claussius-Clayperon equation ?A PV = RT + B/V + y/V2 + ....
B (P + a/V2)(V-b) = RT
C 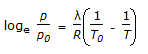
D 
ANS:C - The Clausius-Clapeyron equation describes the relationship between the vapor pressure of a substance and its temperature during phase transitions, particularly the transition from the liquid phase to the vapor phase. The general form of the Clausius-Clapeyron equation is: 𝑑𝑃𝑑𝑇=Δ𝐻vap𝑇⋅Δ𝑉dTdP=T⋅ΔVΔHvap Where:
|


For help Students Orientation
Mcqs Questions
One stop destination for examination, preparation, recruitment, and more. Specially designed online test to solve all your preparation worries. Go wherever you want to and practice whenever you want, using the online test platform.

